cl protons and electrons|How to find the Number of Protons, Electrons, Neutrons for : Pilipinas Chlorine is the 17th element in the periodic table and has a symbol of Cl and atomic number of 17. It has an atomic weight of 35.450 and a mass number of 35. Chlorine has . Pub Casino - #1 in the UK. Pros: 120+ live casino games Low minimum withdrawals 24/7 live chat Cons: Limited poker and baccarat games What our experts think: "Pub Casino's live dealer games make .
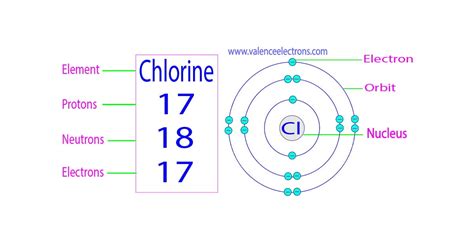
cl protons and electrons,How to find Protons & Electrons for the Chloride ion (Cl-) Wayne Breslyn. 746K subscribers. Join. Subscribed. 260. 26K views 3 years ago. In this video we’ll use the Periodic table and a few.Chlorine – Protons – Neutrons – Electrons – Electron Configuration. Chlorine is a yellow-green gas at room temperature. It is an extremely .These blocks are named for the characteristic spectra they produce: sharp (s), principal (p), diffuse (d), and fundamental (f). Atomic numberThe number of protons in an .cl protons and electronsChlorine is the 17th element in the periodic table and has a symbol of Cl and atomic number of 17. It has an atomic weight of 35.450 and a mass number of 35. Chlorine has . How to find the Number of Protons, Electrons, Neutrons for Chlorine (Cl) - YouTube. Wayne Breslyn. 748K subscribers. 384. 38K views 3 years ago. In this video .How to find the Number of Protons, Electrons, Neutrons for Explanation: Based on the periodic table, the atomic number ( Z of chlorine is 17. Since the atomic number is always equal to the number of protons or. Z = number of .

A neutral chlorine atom has seven electrons in its outermost shell. Only one more electron is needed to achieve an octet in chlorine’s valence shell. (In table salt, .

A neutral chlorine atom has seven electrons in its outermost shell. Only one more electron is needed to achieve an octet in chlorine’s valence shell. (In table salt, .
cl protons and electrons How to find the Number of Protons, Electrons, Neutrons for A neutral chlorine atom has seven electrons in its outermost shell. Only one more electron is needed to achieve an octet in chlorine’s valence shell. (In table salt, .
cl protons and electrons|How to find the Number of Protons, Electrons, Neutrons for
PH0 · What is the electron configuration of Cl
PH1 · Protons, Neutrons, Electrons for Chlorine (Cl, Cl–)
PH2 · How to find the Number of Protons, Electrons, Neutrons for
PH3 · How to find Protons & Electrons for the Chloride ion (Cl
PH4 · Chlorine (Cl)
PH5 · Chlorine
PH6 · 6.1: Ions
PH7 · 4.7: Ions
PH8 · 3.1: Ions